Attributes or combinations of these Some commands accept specifications of nonatomic models **For a quick start, see the examples on the second page of the Chimera Quick Reference Guide (PDF)** In many commands where the specification is the last argumentJJ Thomson's model Plum Pudding model;Ernest Rutherford's model Nuclear model;
Drawing Atoms Montessori Muddle
Model of an atom labeled
Model of an atom labeled-The atomic model must be scientifically accurate! · Main Difference – Thomson vs Rutherford Model of Atom Thomson model of atom is one of the earliest models to describe the structure of atomsThis model is also known as the plum pudding model due to its resemblance to a plum pudding This explains that this atom is a spherical structure made out of a positively charged solid material and the electrons are embedded in that



6 1 Best Atom Diagram Images Stock Photos Vectors Adobe Stock
· The atomic mass (4) is written as a superscript to the left of the element symbol The atomic mass is the sum of the number of protons (2) and the number of neutrons (2) The small atoms that we're looking at tend to have the same number of neutrons as protons, but that's not necessarily the case · Oct 18, 17 See 12 Best Images of Label An Atom Worksheet Inspiring Label an Atom Worksheet worksheet images Drawing Atoms Worksheet Drawing Atoms Worksheet Labeled Parts of an Atom Diagram Label Parts of an Atom Diagram Blank Bohr Model Worksheet · Bohr's Model Of An Atom 1)An atom is made up of three particleselectrons,protons and neutronsElectrons have negative charge,protons have positive charge whereas neutrons have no chargeDue the presence of equal number of negative electrons and positive protons,the atom on the whole is electrically neutral
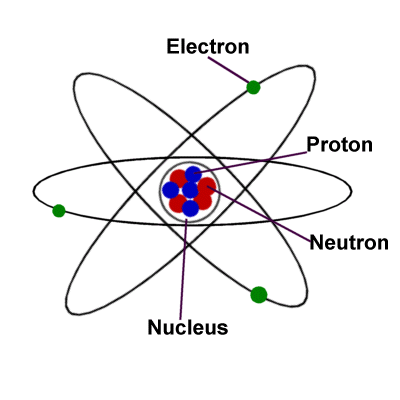
In Bohr's model of a Hyodrogen atom, electrons move in orbits labeled by the quantum number n Find the radius, in meters, of the orbit of an electron around a Hydrogen atom in the n = 5 state according to Bohr's theoryAtomic structure (CCEA) Atoms have a positivelycharged nucleus made of protons and neutrons, and negativelycharged electrons that orbit theMaterialsStyrofoam BallsMetal ringsPaintGlue ThreadTapeI GOT ALL OF THESE AT MICHAELSIt was for my science project All I spent is about $12 I ne
Bohr model of the atom In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus The orbits are labeled by an integer, the quantum number n Electrons can jump from one orbit to another by emitting or absorbing energy · In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3, The sphere n = 1 can accommodate two, the n = Model sulfur atoms are complex, containing nearly 50 parts the Bohr atom model with fixed electrons as a way to simplify atomic structureStart studying Label the parts of an atom Learn vocabulary, terms, and more with flashcards, games, and other study tools



The Structure Of An Atom Explained With A Labeled Diagram Science Struck




The Atom Diagram Quizlet
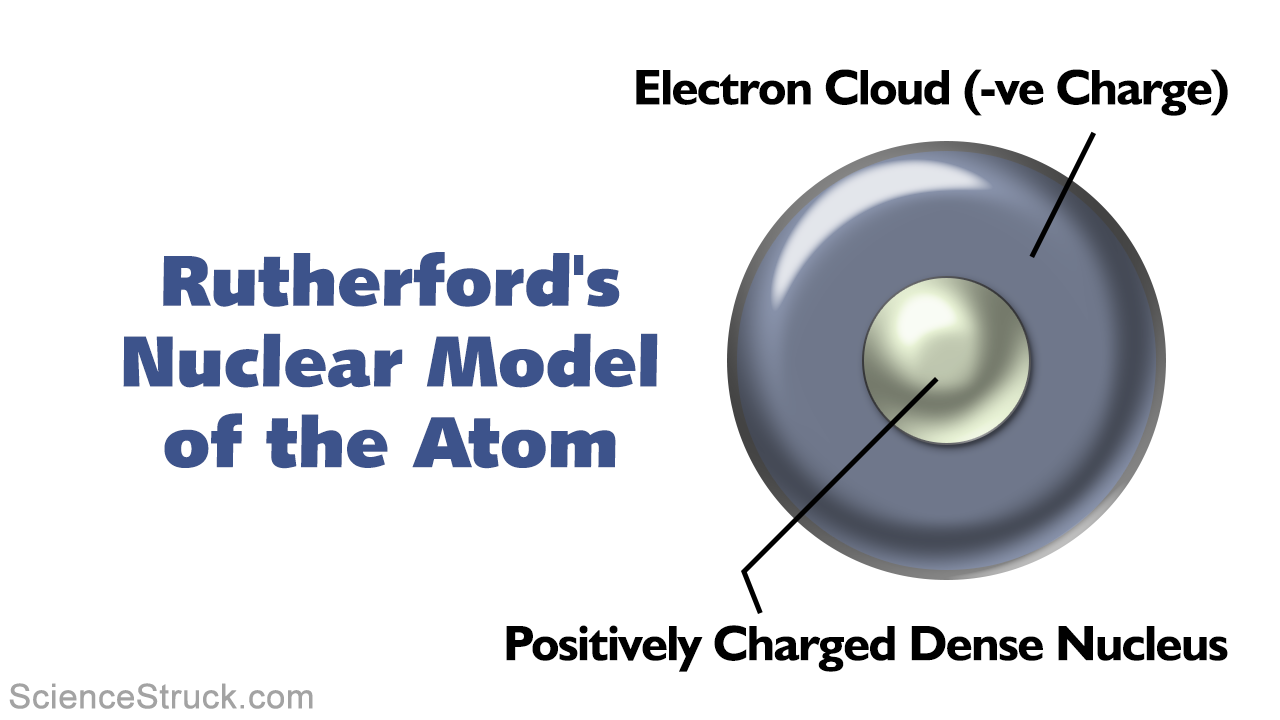
The one experiment that provided the strongest evidence for the nuclear model of the atom is Rutherford's Gold Foil Experiment At Rutherford's labs in Cambridge, England, they set up an experiment where a radioactive particle, an alpha particle, is fired at a very thin sheet of gold7 Have students make a simple drawing of their model of the carbon atom 8 Have students refer to the periodic table of elements to find the chemical symbol for carbon, noting that it is element six and that it has six protons Have students use the periodic table and the same candy pieces to draw and label simple models of the hydrogen,In 1911, a physicist called Ernest Rutherford put forth his idea on the model of an atom Rutherford said that the majority of the atom is empty space In the center was a tiny nucleus, positively charged On the outside, Electrons revolved without pattern around the nucleus, the space in between the electrons and the nucleus being empty



What Is Electricity Learn Sparkfun Com




Atom Definition Structure History Examples Diagram Facts Britannica
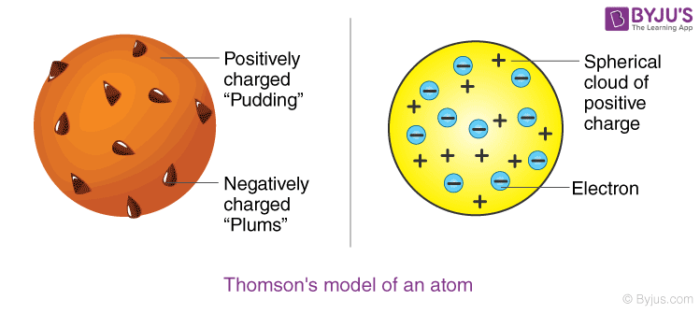
First week only $499! · When JJ Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom According to Thomson Model of an atom 1)An atom consist of a sphere of positive charge with negatively charged electrons embedded in it 2)The positive and negative charges in an atom are equal in magnitude,due toElectrons are thought to inhabit areas of probability ID XCXEN8 (RF)




What Are The Parts Of An Atom Universe Today



Development Of Atomic Theory
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravityFigure 85 "Bohr's Model of the Hydrogen Atom" shows a model of the hydrogen atom based on Bohr's ideas Figure 85 Bohr's Model of the Hydrogen Atom (the one labeled d z 2 has two lobes and a torus instead of four lobes, but it is equivalent to the other orbitals) When there is more than one possible value of mParts of an atom table and labeling worksheet with answer key by EZ Science $150 Word Document File Students have to fill in a data table about the parts of an atom and then label a picture of an atom Answer key is included Subjects Science, Other (Science), General Science



31 Label The Parts Of An Atom Labels Database
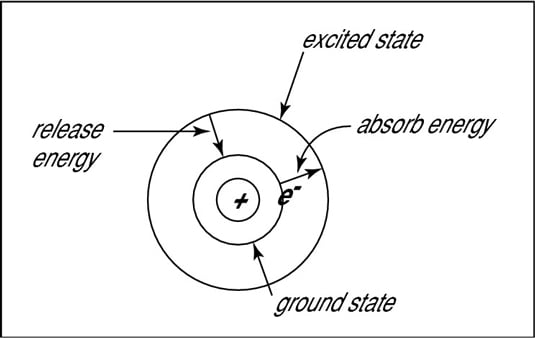



Atomic Structure The Bohr Model Dummies
The first atom I build with the kids is carbon (atomic #6), which is done for them on the Atom Model Demonstration Worksheet I add 6 protons (count out loud in a dramatic fashion) and 6 neutrons to the nucleus, explaining that the proton count represents the atomic number and the total contents of the nucleus (hamster ball) represents the atomic massCh 2 Homework Label Each Model Of An Atom With Its Appropriate Information 3 Phosphorus 05 Oxygen o Sulfur Reset ZoomAtom labeled diagram 600 x 429 Download png 26 kb transparent png 36 kb



Test Physcial Science
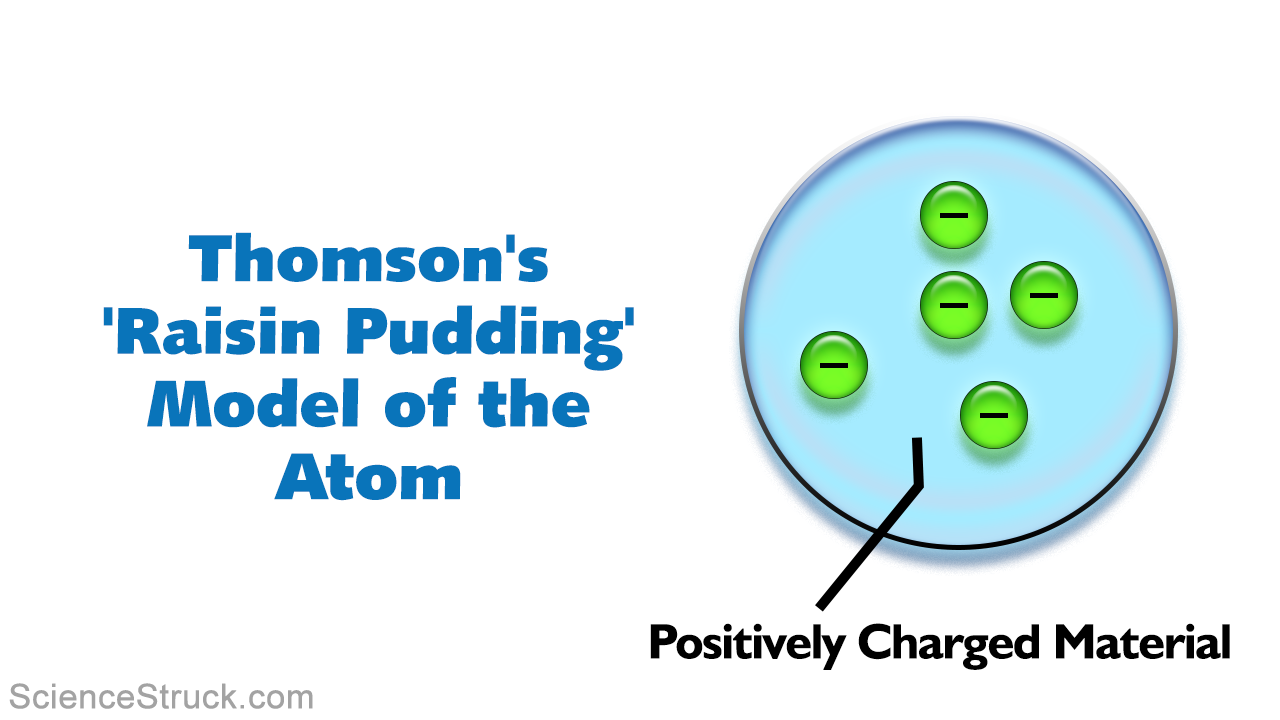



The Structure Of An Atom Explained With A Labeled Diagram Science Struck
A British scientist named J J Thomson discovered the electron in 17 He suggested that the atom looked like the model below, with electrons in a mass, or cloud, of positive charge The Bohr model showed electrons surrounding the positive nucleus Calcium is an element · The model should include an attached information tag (properly labelled) that clearly identifies the Name of an element and its atomic symbol, atomic mass with subatomic particles 3D ATOMIC MODEL RUBRIC CRITERIA OUTSTANDING (9) SATISFACTORY (6) NEEDS IMPROVEMENT (3) MODEL ACCURACY Atom is accurate and included all required informationAlthough the most commonly used model of the atom is the Bohr model, scientists are still developing new and improved theories on what the atom looks like One of the most important contributions to atomic theory (the field of science that looks at atoms) was the development of quantum theory




Models Of The Atom The Atom Siyavula




Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels
Atom Specification Atoms can be specified in commands using hierarchical specifiers;Conceptual computer artwork representing the structure of an atom Eight electrons are orbiting the central nucleus along definite paths This is a schematic 'Bohr model' of an atom;Draw and label a simplified model of an atom Explain how this model misrepresents our understanding of atomic structure close Start your trial now!




Partially Labeled Pov Ray In Ball And Stick Model Diagram Showing The Download Scientific Diagram




The Structure Of An Atom Explained With A Labeled Diagram Science Struck
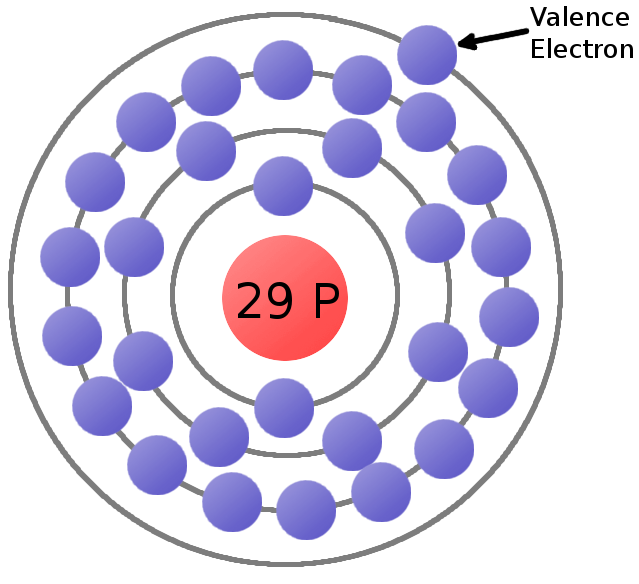
Every element is unique and has an atomic number That number tells you the number of protons in every atom of the element The atomic number is also called the proton number Charges of Atoms You can see that each part of the atom is labeled with a "", "", or a "0" Those symbols refer to the charge of the particle* The model should have the proper number of protons, neutrons and electrons The atomic model must be properly labeled ASSESSMENT Your model will be graded on the following criteria creativity, imagination and scientific accuracy Your model will be a major exam gradeLabel each model of an atom with its appropriate Information oxygen 0 nitrogen "N os carbon с phosphorus hydrogen H sulfur Electron configuration Below is a diagram illustrating electron shells around a chloride ion Keeping in mind that chloride has an atomic number of 17, drag the appropriate number of electrons to each electron shell to



Chem4kids Com Atoms Structure




What Are The Parts Of An Atom
· Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established This picture was called the planetary model, since it pictured the atom as a miniature "solar system" with the electronsIn the space provided, describe the locations of the subatomic particles in the labeled model of an atom of nitrogen below, and give the charge and relative mass of each particle a proton The proton, a positive and relatively massive particle, should be located in the nucleusAn atomic model is a theory trying to explain the structure of the atom When the exact structure of the atom remained quite unknown, models were proposed based on experimental evidence of the properties of matter Such models would be purely theoretical constructs However, they should be able to explain the basic properties of matter




Drawing Atoms Montessori Muddle




33 Draw And Label The Parts Of An Atom Labels For Your Ideas
Labelled Diagram Of An atom the structure of an atom explained with a labeled diagram an atom is the smallest building block of all matter made up of neutrons protons and electrons atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus atoms diagrams electron configurations ofJcjdjsjdisososososoo is waiting for your help Add your answer and earn pointsArrow_forward Question Draw and label a simplified model of an atom Explain how this model misrepresents our understanding of atomic structure check_circle Expert
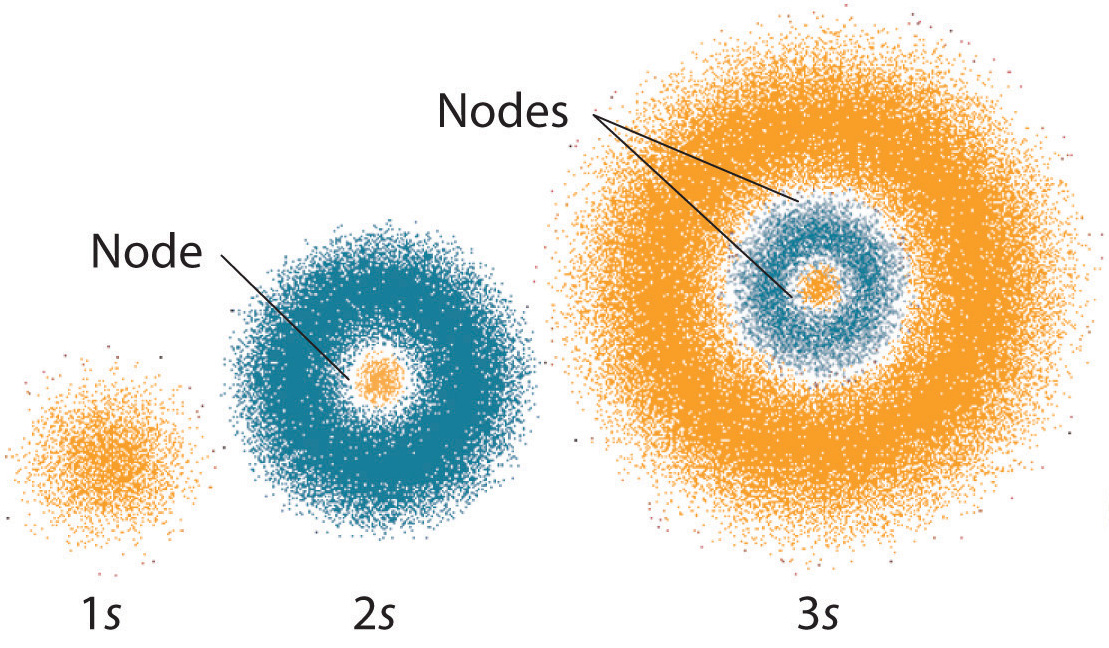



The Quantum Mechanical Model Of The Atom Article Khan Academy



What Are The Parts Of An Atom
Feb 28, 18 Atom Model with Protons, Electrons and Neutrons of millions of high resolution stock photos, illustrations and vectors See more ideas about atom model, atomic structure, atom1 Log in Join now 1 Log in Join now Middle School Biology 5 points savage Asked The model shows parts · The Rutherford model is one of the most popular models of the atom even though it was only considered accurate from 1909 to 1913 Notable Terms Nucleus The nucleus is the center of an atom with




6 1 Best Atom Diagram Images Stock Photos Vectors Adobe Stock




Atoms And Elements Biology For Non Majors I
· When we talk concerning Label an Atom Worksheet, we've collected particular variation of photos to inform you more drawing atoms worksheet, blank bohr model worksheet and labeled parts of an atom diagram are three of main things we will show you based on the gallery title Beside that, we also come with more related ideas like label parts of anLabel Parts Of An Atom Displaying top 8 worksheets found for Label Parts Of An Atom Some of the worksheets for this concept are Parts of an atom work answers, Name section parts of the atom, North paul maplewood oakdale overview, Blank diagram of atom to label, Atomic structure work, Chemistry of matter, Parts of a microscope s, An atom apart · We draw atomic structures for any element with the help of atomic number they have So, if you want to draw atomic structure for sodium first of all you should know the atomic number for sodium Atomic number of sodium is 11 Atomic number of any element refers to the number of electrons in an atom of that element they are having STEP 1 Always write the atomic




Democritus Atomic Model Labeled 618x370 Png Download Pngkit




Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com
The ancient Greek philosopher Demokritos ( BCE) thought that matter was made up of millions of tiny, uncuttable pieces of that same matter In fact, the word atom · Click here 👆 to get an answer to your question ️ The model shows parts of an atom What term identifies the parts of the atom labeled with a Y? · The first person who discovered electrons, Sir JJ Thompson, put forth his 'Plum Pudding' Model of an atom He believed that the atom was a uniform sphere with a positive charge and had electrons embedded in it He could explain the electrical neutrality of an atom, but could not explain the arrangement of the fundamental particles in an atom




Label The Atom Diagram Quizlet
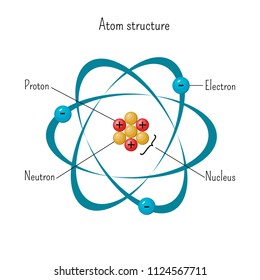



Atom Diagram Hd Stock Images Shutterstock
Alternative Titles Rutherford atomic model, nuclear atom, planetary model of the atom Rutherford model, also called Rutherford atomic model, nuclear atom, or planetary model of the atom, description of the structure of atoms proposed (1911) by · Which model of an atom is correctly labeled?Niels Bohr's model Planetary model;




Which Model Of An Atom Is Correctly Labeled Brainly Com




What Is An Atom Definition Overview Expii
As such, there are five basic atomic models which have helped us define and understand the structure of the atom more clearly The five atomic models are as follows John Dalton's atomic model Dalton´s Billiard Ball (Solid Sphere) Model;
:max_bytes(150000):strip_icc()/Thallium-58b6010d5f9b5860464bac8b.jpg)



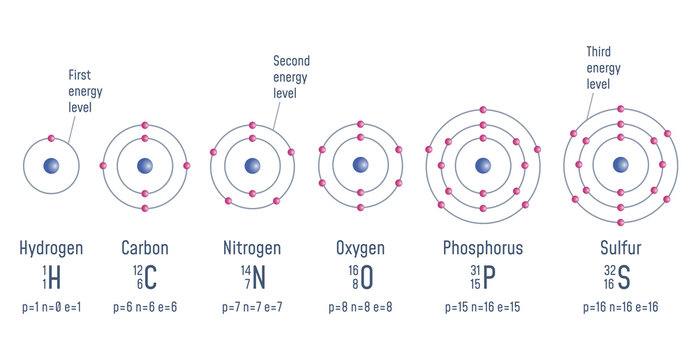
Atom Diagrams Electron Configurations Of The Elements




Snc1p
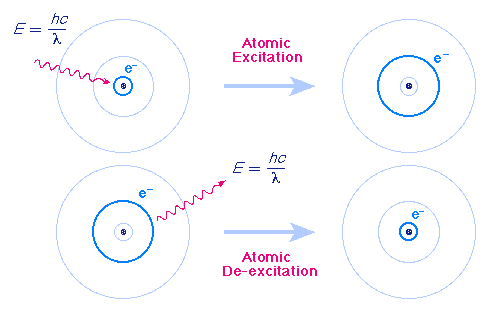



The Bohr Model
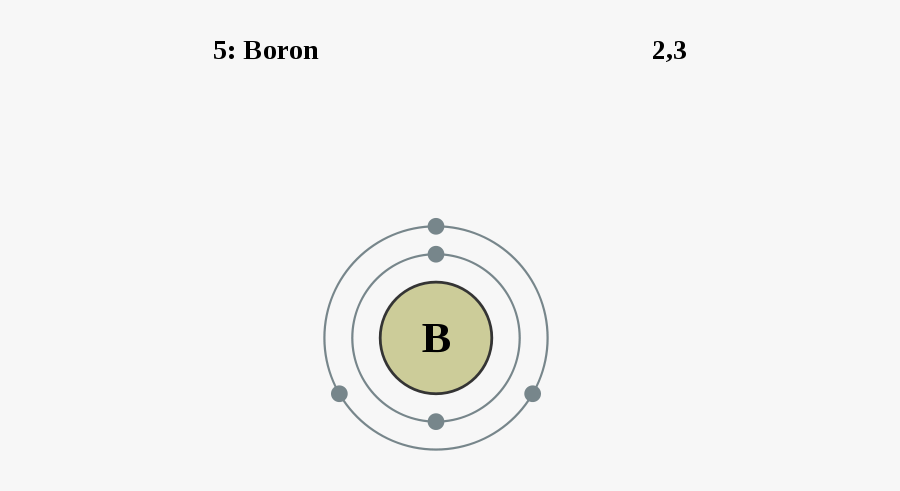



Labelled Diagram Of An Atom Of Boron Free Transparent Clipart Clipartkey
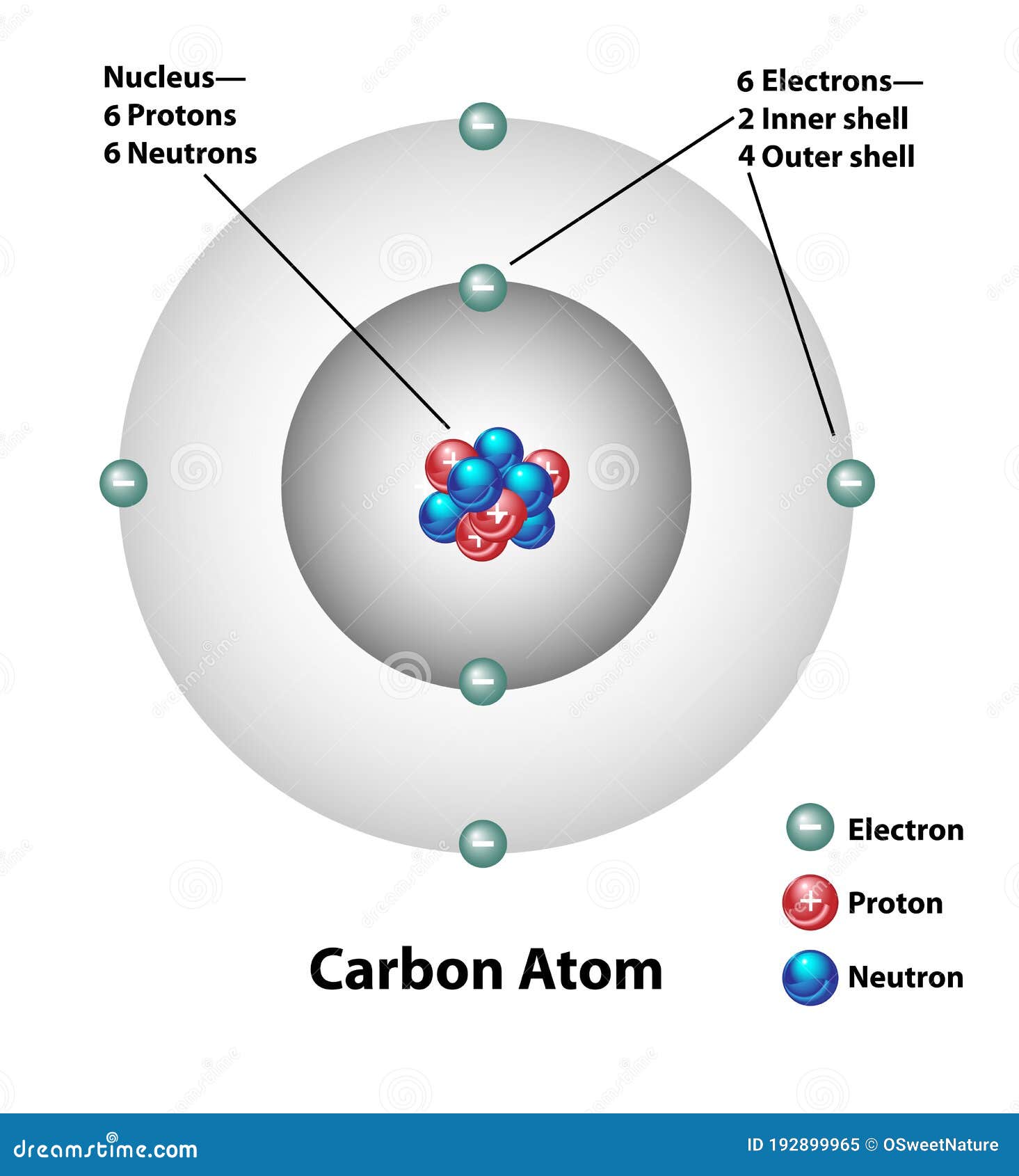



Carbon Atom Molecular Structure Labels Stock Vector Illustration Of Labels Diagram




Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets



What Is The Difference Between The Solid Atom In Dalton Atomic Theory And Thomson Atomic Model Quora



Changes In Dalton S Atomic Theory Dalton S Atomic Theory




Diagram Of Boron Atom Labeled Wiring Diagram Value




Diagram Of The Atom Science Projects Projects For Kids Atom
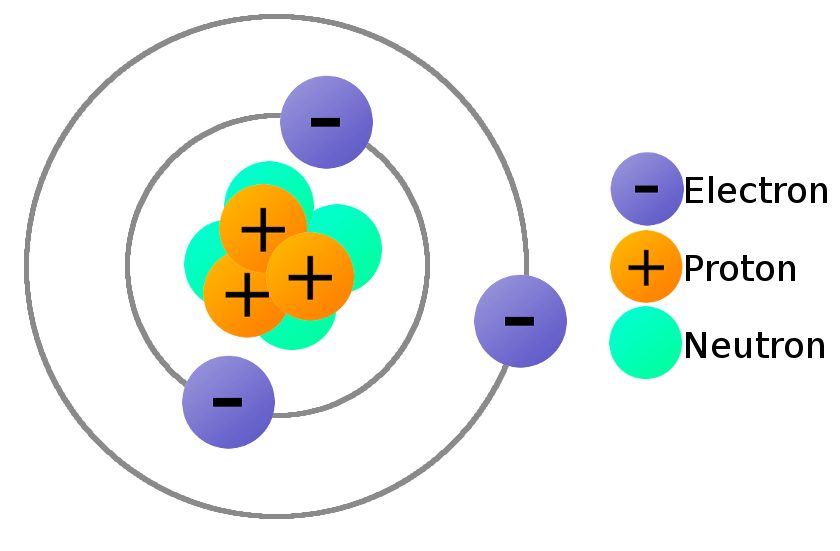



What Is Electricity Learn Sparkfun Com




Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com




Bohr S Model Of An Atom With Postulates And Limitations Byju S
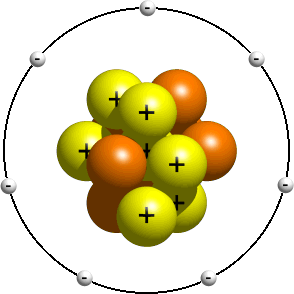



Questions And Answers How Do I Make A Model Of An Atom




Atoms To Molecules Read Earth Science Science Chemistry Atom Diagram Teaching Chemistry




Bohr Model Description Development Britannica




The Development Of The Atomic Model Wired




Labeled Diagram Of Magnesium Atom Wiring Diagrams Lose




Clip Art Atom B W Labeled I Abcteach Com Abcteach




3d Render Of Atom Structure Of Aluminum Isolated Over White Background Protons Are Represented As Red Atom Diagram Atom Model Atom Model Project
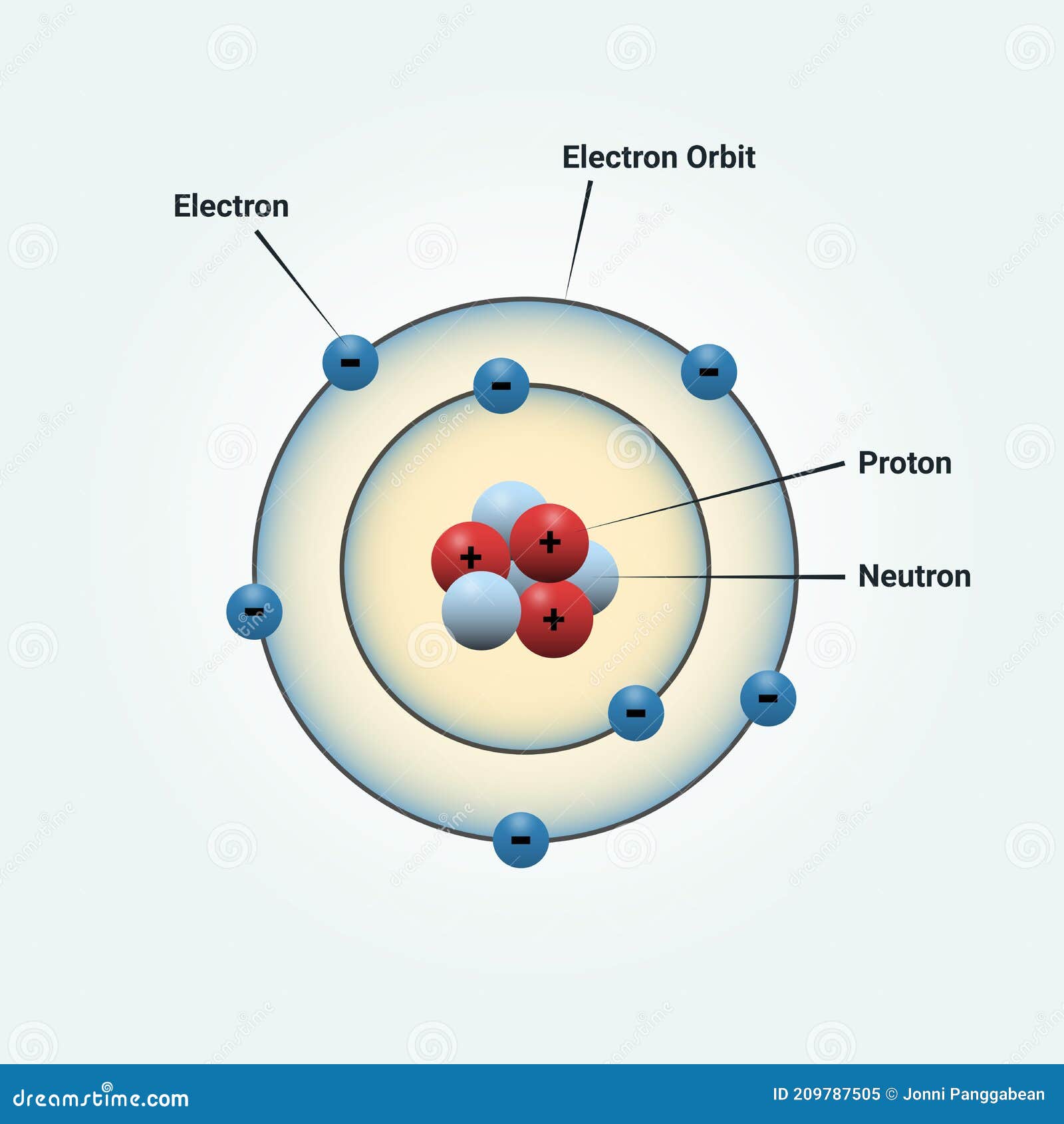



Nitrogen Atom Diagram Stock Illustrations 48 Nitrogen Atom Diagram Stock Illustrations Vectors Clipart Dreamstime
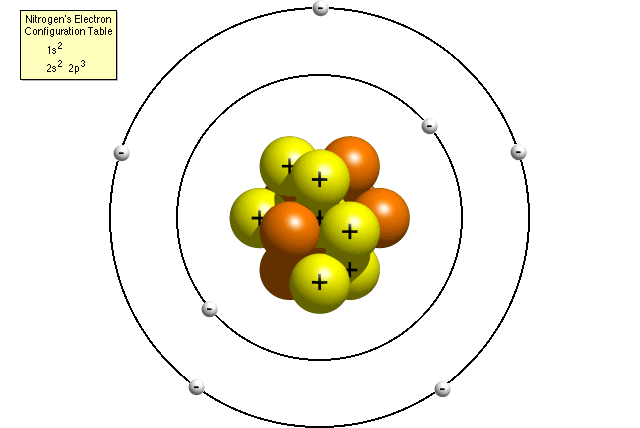



Questions And Answers How Do I Make A Model Of An Atom



Thomson Atomic Model Plum Pudding Model Postulates Limitations




Q5 Draw A Neat Labelled Diagram Representing An Atom Name Th Lido



Science For Kids The Atom




Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com




What Is An Atom It S A Question Of Physics The Atomic Age Linda Hall Library Kansas City Mo




Atom Diagram Universe Today
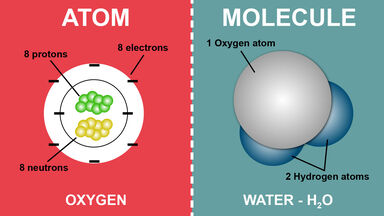



Basic Difference Between An Atom And A Molecule
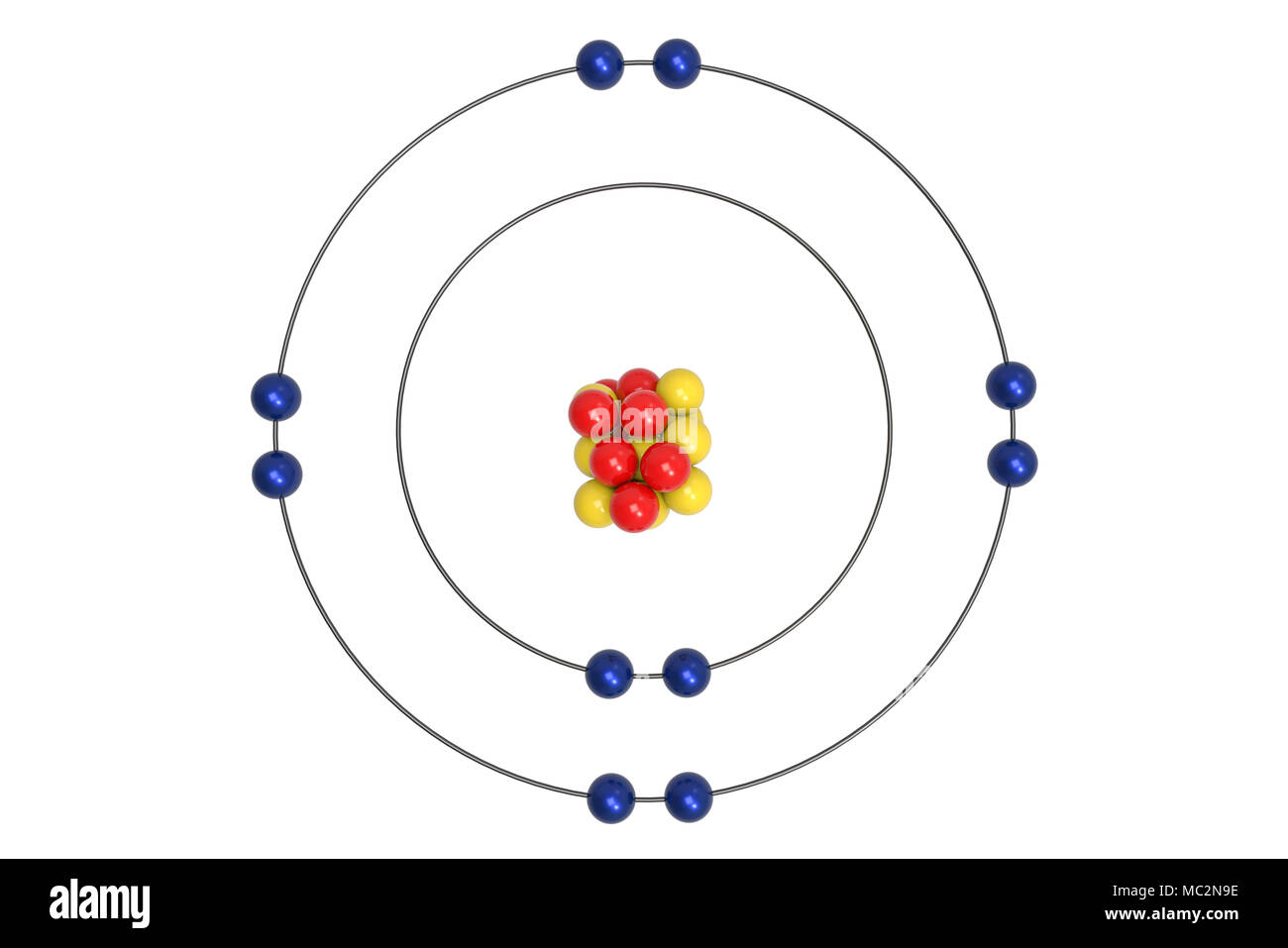



Neon Atom High Resolution Stock Photography And Images Alamy
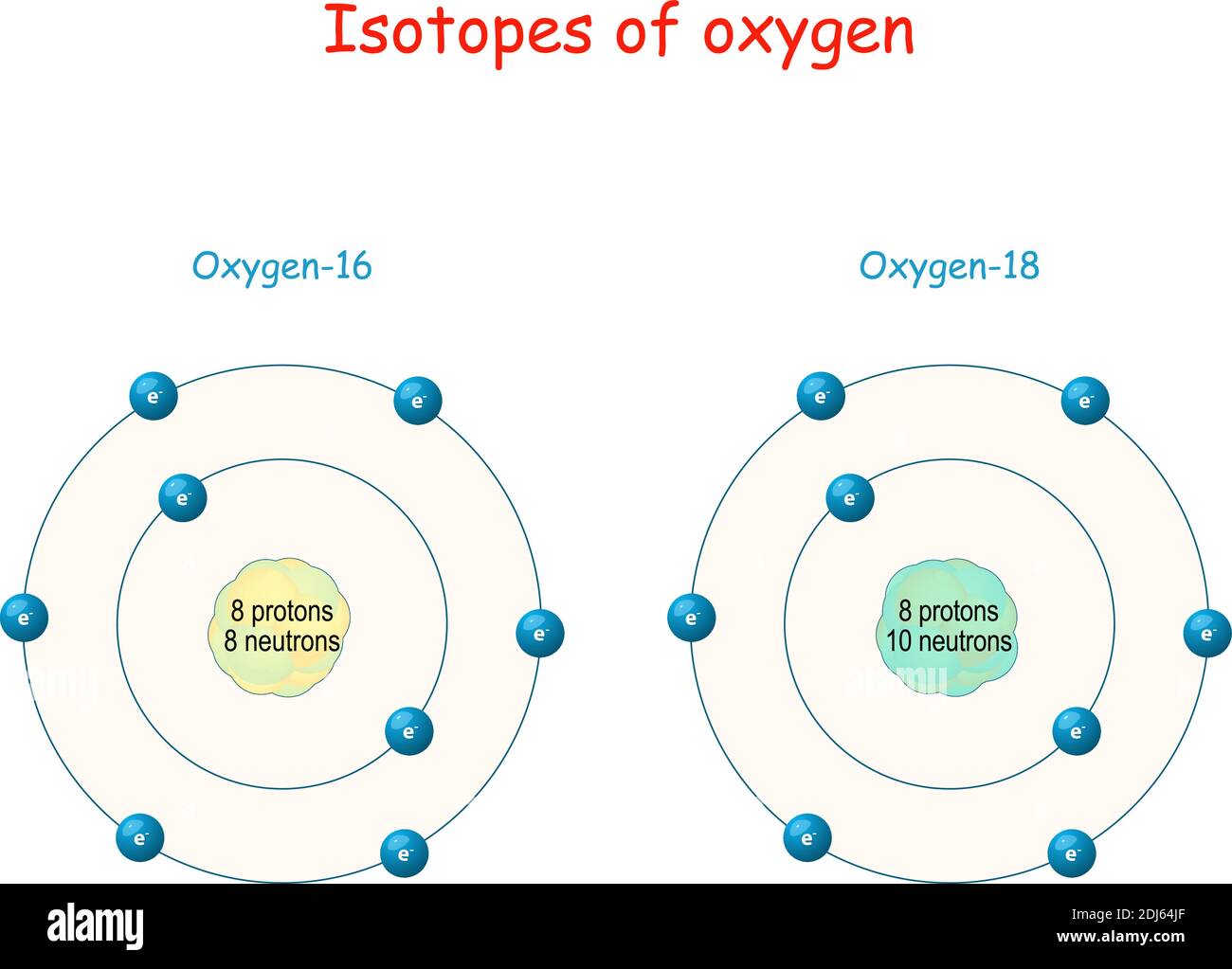



Proton Neutron Protons Neutrons High Resolution Stock Photography And Images Alamy
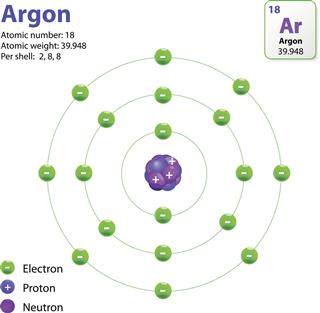



The Structure Of An Atom Explained With A Labeled Diagram Science Struck
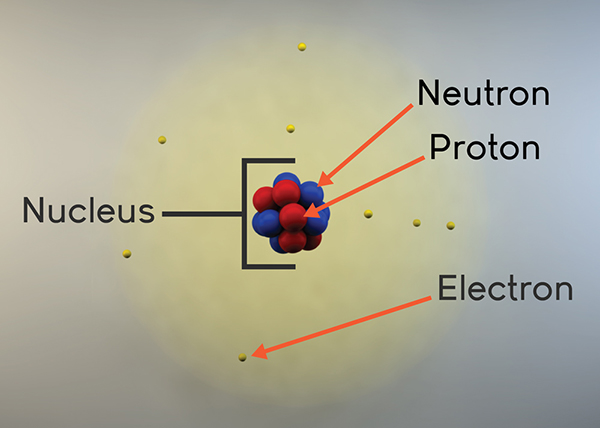



Models Of The Atom Animation Annenberg Learner



Bohr Atomic Model
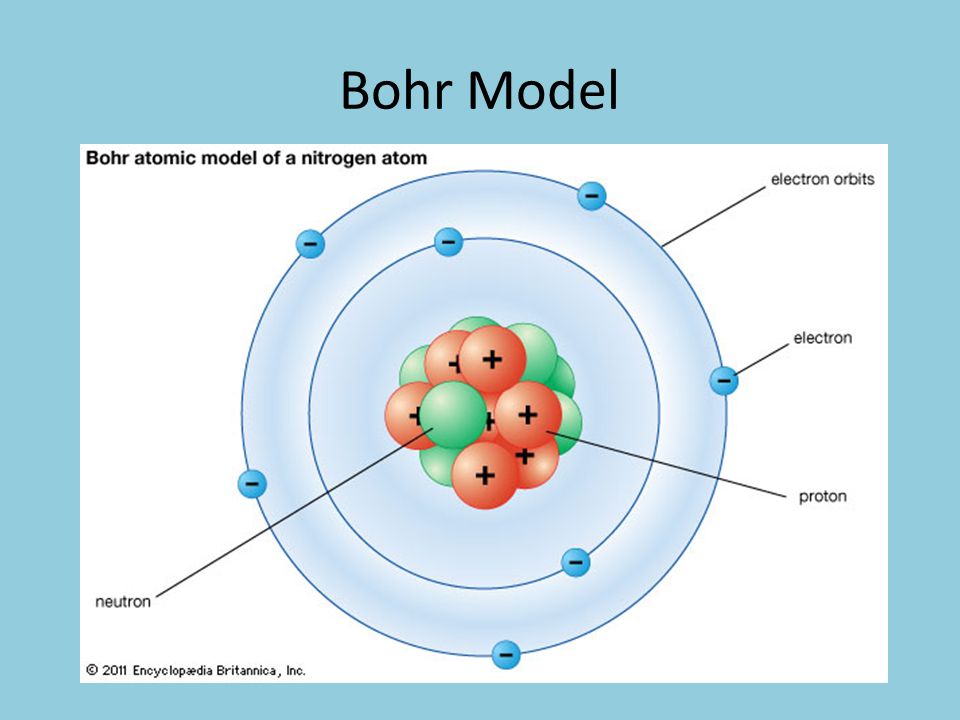



Unit 5 Electrons In Atoms Ppt Video Online Download



Molecular Structure Carbon Atom Electrons Protons Stock Vector Royalty Free
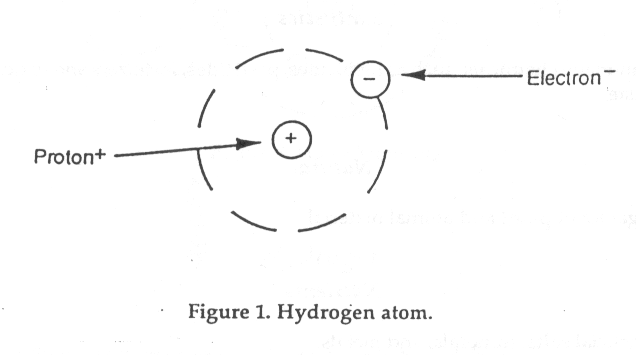



Labeled Diagram Of Hydrogen Atom Wiring Blog Diagram Data




Bohr Model Wikipedia




Manhattan Project Exploring The Atom 1919 1932
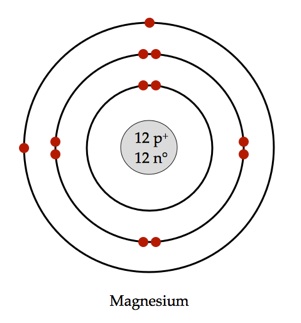



What Would A Bohr Model For Magnesium Look Like Socratic
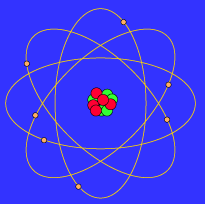



The Bohr Model
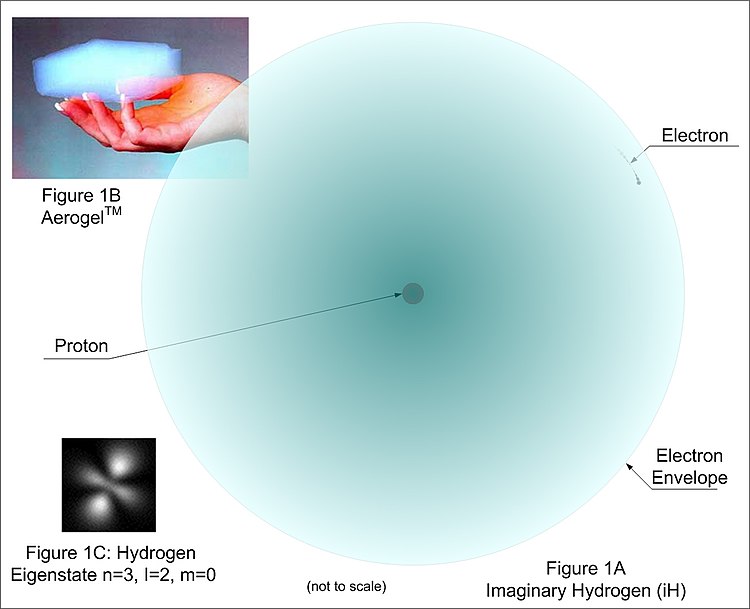



A New Model Of The Atom Wikibooks Open Books For An Open World



Dalton S Atomic Theory Read Chemistry Ck 12 Foundation




The Development Of The Atomic Model Wired



Boron2 The Bohr Rutherford Model Helium Atom Atomic The Flickr




Modern Model Atomic History




Bohr Model Description Development Britannica




Models Of The Atom The Atom Siyavula



Rutherford Model Wikipedia



31 Label Of An Atom Labels Database




Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels



Drawing Atoms Montessori Muddle



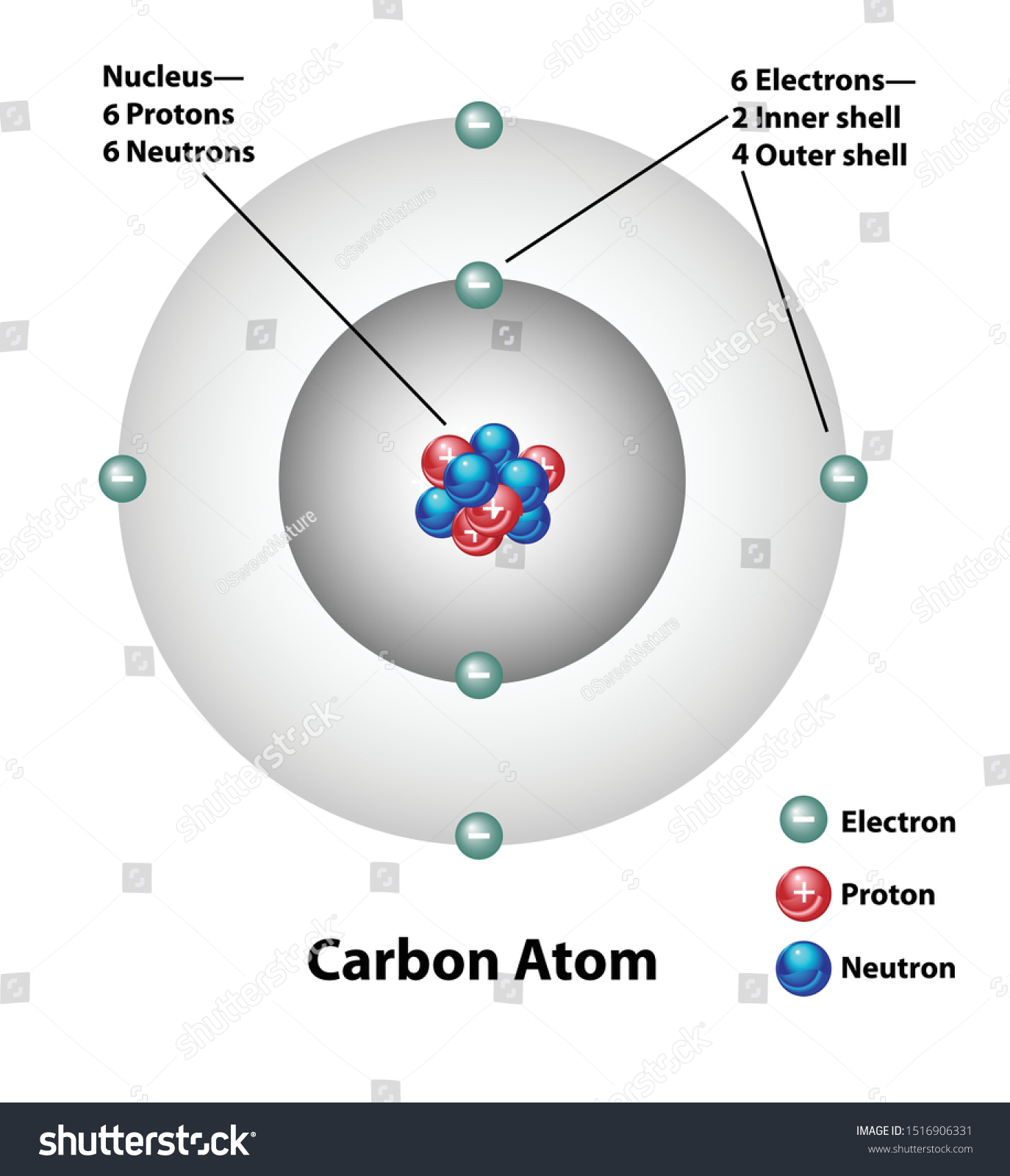
Sample 6 Chemistry Carbon Atom Accessible Image Sample Book



The Structure Of An Atom Explained With A Labeled Diagram Science Struck




Atom Definition Structure Parts With Labeled Diagram
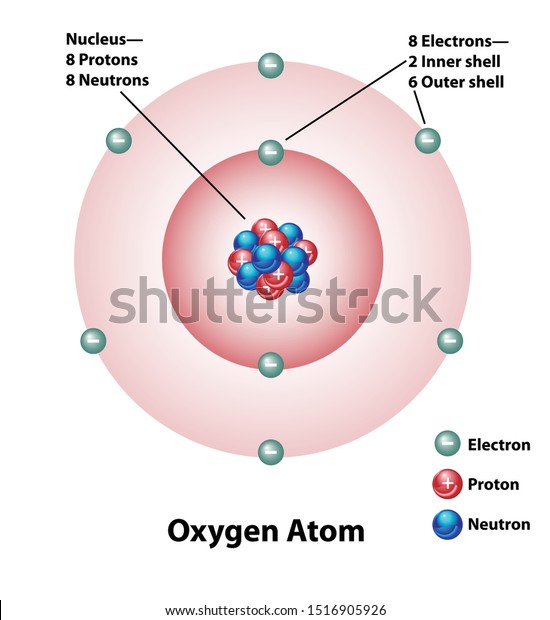



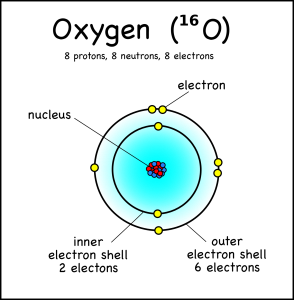
Diagram Oxygen Atom Nucleus Inner Outer Stock Vector Royalty Free
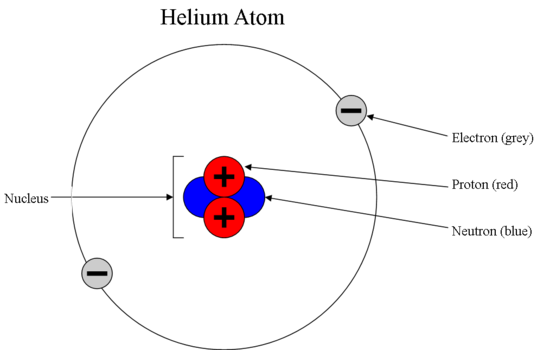



Sub Atomic Particles Chemistry Libretexts
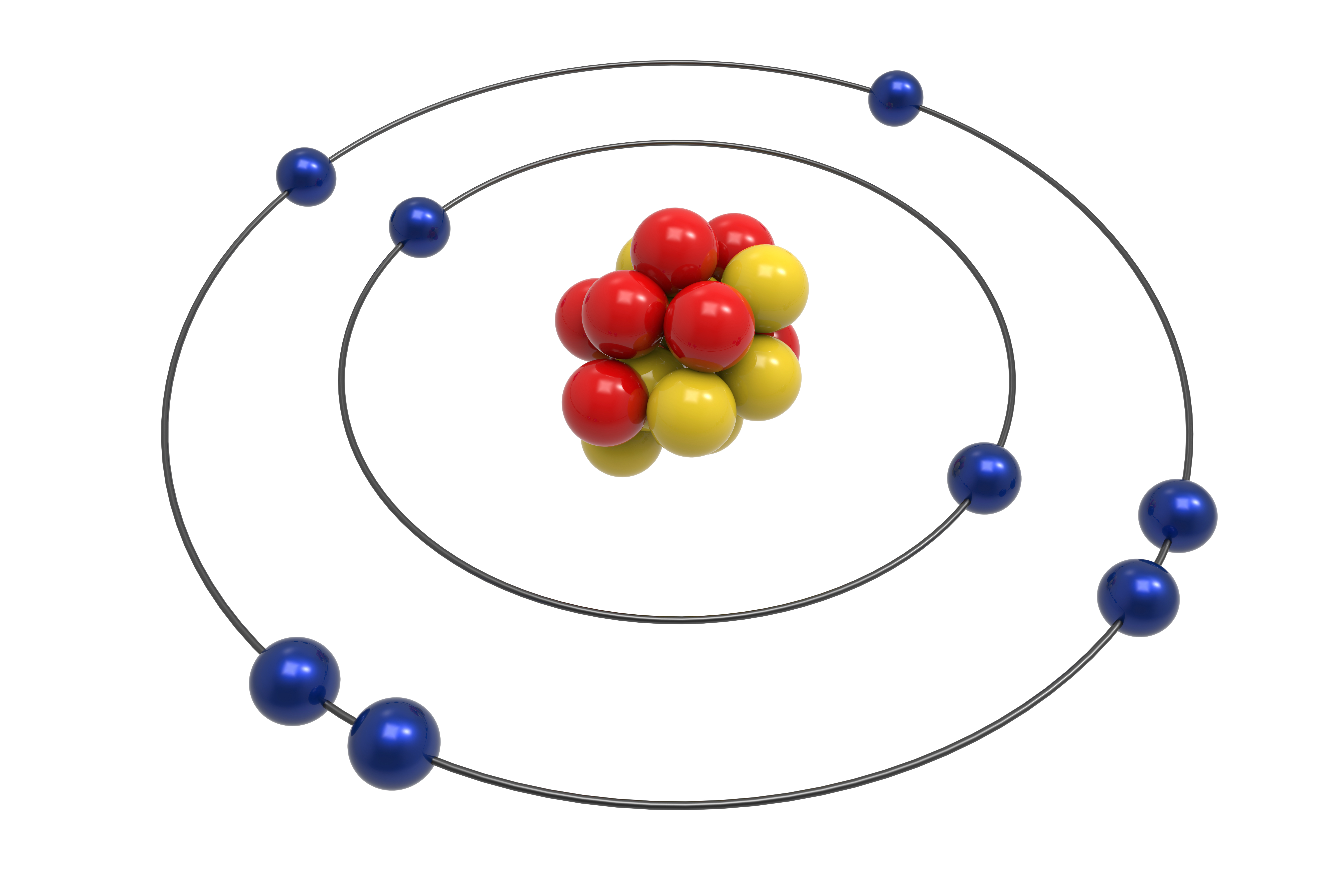



What Are The 4 Atomic Models




Neon Atomic Structure Stock Image C013 1512 Science Photo Library



Drawing Atoms Montessori Muddle




The Best 12 Atom Labeled With Protons Neutrons And Electrons
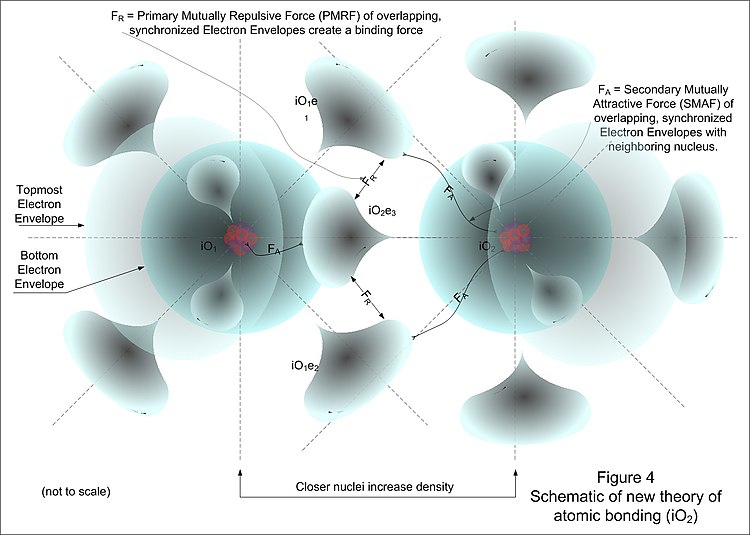



A New Model Of The Atom Wikibooks Open Books For An Open World




Thomson Atomic Model Description Image Britannica




Diagram Of Phosphorus Atom Atom Diagram Atom Model Atom Model Project




Models Of The Atom The Atom Siyavula




The Structure Of The Atom Astronomy
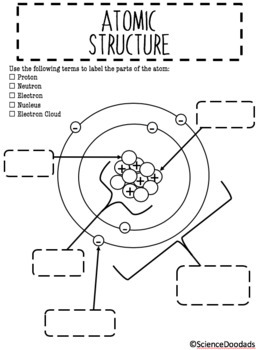



Atomic Structure Labeling Diagram By Science Doodads Tpt
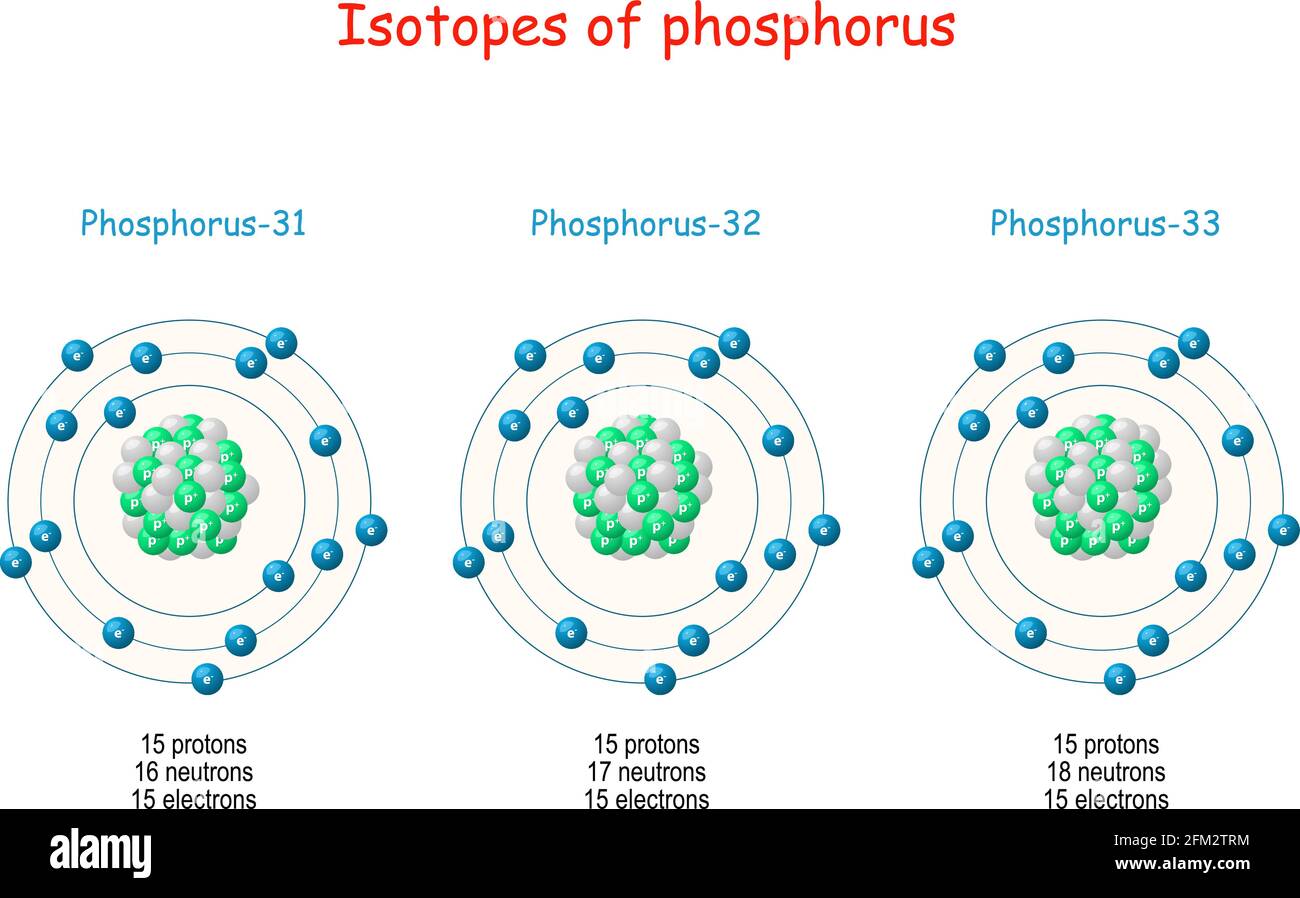



Proton Neutron Protons Neutrons High Resolution Stock Photography And Images Alamy




Bohr Diagram Of Sodium Atom Diagram Atom Model Atom Model Project
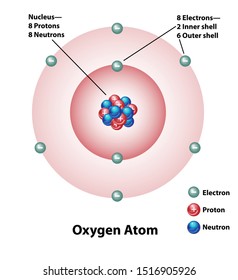



Atom Diagram Hd Stock Images Shutterstock




Q5 Draw A Labelled Diagram Of An Atom Including Its Nucleus Lido




Phosphorus Atomic Structure Stock Image C013 1528 Science Photo Library



0 件のコメント:
コメントを投稿